Chimeric antigen receptors, or CARs, are synthetic proteins that can re-direct T cells to robustly target and kill cancer cells. Early clinical trials revealed impressive results in the treatment of B cell leukemia and lymphoma, representing a breakthrough in the treatment of these diseases with historically poor outcomes. Despite promising early results, long-term follow-up has revealed that many patients will not respond to these therapies, and that many who do respond will ultimately relapse.
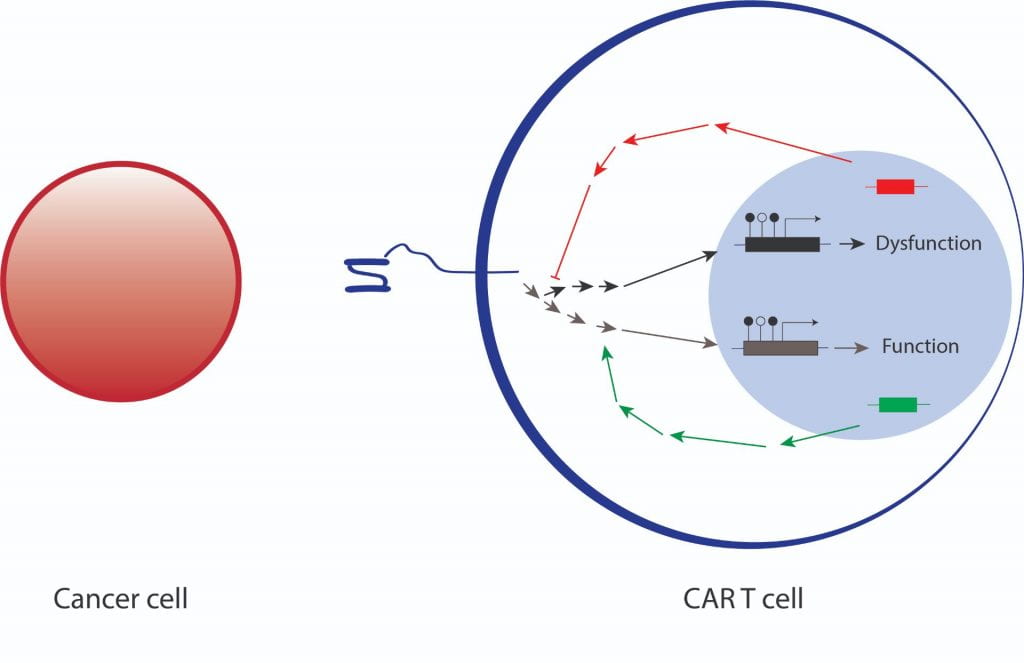
The Singh Lab recently discovered that, under certain conditions, CARs can cause T cells to fail, instead of directing them to kill cancer cells. Using a variety of classical and novel techniques, we aim to understand how CARs in some cases drive effective T cell function against cancer, and in others lead to the development of T cell dysfunction that prevents successful anti-cancer activity. From basic receptor biochemistry and signaling to epigenetic imprinting, we hope to develop a blueprint of how these synthetic receptors direct T cell molecular programming.
Using knowledge of these essential pathways, we will use novel genome engineering techniques to overcome detrimental cellular circuitry and develop enhanced, “intelligent” cellular therapies for cancer that are more effective, more durable and yield more long-term remissions for patients with cancer.
Current Research
Research centers on understanding how CARs direct T cell molecular programming to either promote or inhibit successful anti-cancer function. This work is based on understanding which intracellular signaling pathways are activated by CARs (and how “strongly” they are activated) as well as on how these pathways instruct cellular activity in the form of protein activation, gene transcription and epigenetic regulation.
Currently, two types of CARs have entered clinical practice: those engineered with a CD28 co-stimulatory domain, and those with a 41BB co-stimulatory domain. We have made several observations that reveal fundamental differences in the T cell biology driven by these different CARs. Using advanced molecular techniques, including phospho-enriched mass spectrometry, single-cell sequencing and CRISPR-based genome engineering, we are investigating how these two CARs can control effective T cell function against cancer, or alternatively drive the development of T cell dysfunction that leads to therapeutic failure.